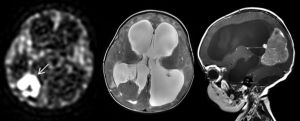
Pediatric brain tumors are the number one cause of cancer-related deaths in children.
Childhood cancer is the leading cause of death beyond infancy due to disease in the United States, with leukemia as the most common cancer. From 1975 to 2013, 5-year survival rates for childhood cancer have improved from just over 50% to 83%, primarily from an improvement in the treatment of leukemia and lymphomas. As a result, pediatric brain tumors are now the most common causes of cancer-related mortality, in addition to being the most common solid tumors in children. Five hundred and thirty-four children died of brain cancer in the United States in 2014.1 Compared with adult brain tumors, pediatric tumors as a heterogeneous group have a better prognosis; however, significant comorbidities are being recognized in survivors, including greater risks of hypertension, myocardial infarction, stroke, and type 2 diabetes, as well as decreased levels of cognitive and social function, with an overall decreased quality of life.2 As a group, the incidence of pediatric CNS tumors is 5.54 per 100,000, and an estimated 3560 new patients are expected to be diagnosed in the United States in 2018.3
The quantification of radiographic phenotypes will improve radiomics.
Radiomics, particularly in the field of oncologic imaging, is a necessary evolution to keep pace with the seemingly rapid advances in the field of molecular genomics of individual cancers. Understanding specific molecular expressions of a patient’s tumor allows more targeted therapy that would be more effective and potentially less toxic. Radiographic exams of pediatric brain tumors are not just images; they represent data to be quantified, and with computational help, to be used in a multiparametric approach to serve as specific imaging biomarkers for tumor genotypes and molecular expressions. This field of radiomics will improve diagnosis by predicting not only tumor type, but also subtype and prognosis, as well as guiding surgical approach, particularly when gross total resection is not safely achievable. The remainder of this introduction will focus on advanced techniques that are applicable for the quantification of all pediatric brain tumors, as well as specific advances in radiomic knowledge for each of the most common tumor types.
Diffusion imaging remains a mainstay of tumor grading.
DWI and resulting ADC are well-accepted methods of differentiating high-grade and low-grade tumors, likely based on an inverse relationship between ADC and tumor cellularity. Koral et al4 systematically evaluated the added value of DWI and ADC in pediatric cerebellar tumors, where 5 out of 6 reviewers showed a significant improvement in diagnosis with DWI/ADC and the correct diagnosis increased by greater than 3-fold. Vajapeyam et al5 combined ADC histograms and dynamic contrast enhancement (DCE) permeability in a multiparametric approach with a combined ROC analysis of 0.92 area under the curve. Rodriguez Gutierrez et al6 used ADC map texture features and resulting histogram analysis in a machine-based classification, which showed greater than 91% accuracy for all of the included pediatric posterior fossa tumors and was able to discriminate 89.4% of classic histologic medulloblastomas from nonclassic medulloblastomas. Finally, multiple-b-value diffusion modeling (intravoxel incoherent motion) has also shown promise in differentiating high- and low-grade tumors with parameters such as the true diffusion coefficient and pseudodiffusion, which correlates with capillary perfusion.7
Perfusion techniques can differentiate high- and low-grade tumors.
Given their high metabolic demand, higher grade tumors have a propensity for neoangiogenesis and vascular recruitment, leading to a greater blood volume and flow relative to the normal white matter. This has been typically investigated with DSC, which utilizes a bolus of gadolinium with T2*-weighted echo-planar imaging for the quantification of first-pass bolus effects. Our group has published one of the largest DSC studies on pediatric brain tumors, finding that the use of relative cerebral blood volume (rCBV) had high sensitivity and negative predictive value in discriminating low and high tumor grades, but is limited in specificity. We concluded that the use of rCBV in routine practice for experienced pediatric neuroimagers may be helpful in cases when conventional imaging is nonspecific.8
DCE is a T1-based perfusion technique that maps the rise in T1 signal over time as gadolinium washes into the tumor, with permeability as a primary quantified measure. In addition to the multiparametric approach with ADC, Vajapeyam et al9 found higher grade tumors to have higher transfer constants (Ktrans) and rate constants (Kep), but lower extravascular extracellular volume fractions (Ve). All 3 parameters achieved high specificity (82–100%), with Ve having the highest sensitivity (71%).
With the current literature demonstrating linear gadolinium brain deposition, the use of lower relaxivity macrocyclics may be safer, or it may be better yet to utilize perfusion techniques without contrast at all. Yeom et al10 used 3D pseudocontinuous ASL to significantly differentiate high- and low-grade tumors using relative tumor blood flow.
Medulloblastoma subtypes are further classified by risk stratification.
The subtyping of medulloblastomas into 4 different molecular pathway subgroups—WNT, SHH, group 3, and group 4—each with a differing tumor behavior and prognosis, has changed how we view the most common malignant pediatric brain tumor. This was recently summarized by Dr. Marlene Baumann in the November–December 2017 AJNR News Digest. However, even this recent classification system was modified by Ramaswamy et al11 according to risk stratification into low risk (>90% survival), standard risk (75–90%), high risk (50–75%), and very high risk (<50%) for childhood medulloblastomas outside of the infantile subgroup. WNT and nonmetastatic group 4 tumors with whole chromosome 11 loss or 17 gain are low-risk and may qualify for less toxic therapy. Standard-risk tumors include nonmetastatic, non-TP53, non-MYCN-amplified SHH, and non-MYCN-amplified group 3 and 4 without chromosome 11 loss. High-risk tumors include metastatic SHH or group 4 tumors, as well as MYCN-amplified SHH. Finally, very high-risk tumors include group 3 with metastases or SHH with TP53 mutations. Further correlation of radiographic tumor types with these risk groups would be helpful to predict prognosis and guide treatment.
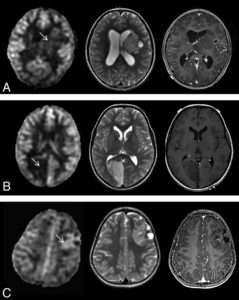
Diffuse intrinsic pontine gliomas showing decreased diffusion have a shorter survival.
Diffuse intrinsic pontine glioma (DIPG) continues to have one of the worst prognoses for pediatric brain tumors, with a median survival of 6 months and no effective treatment options. Recent discoveries of histone mutations (H3.1 or H3.3, position K27) of midline gliomas may help develop targeted therapy for this devastating disease. Aboian et al12 described radiographic phenotypes of histone H3K27-mutated midline gliomas, which were located in the pons, thalamus, vermis, and spinal cord. They described various appearances, from nonenhancing expansile infiltrative masses to areas of central necrosis and peripheral enhancement with little adjacent T2 hyperintensity, which did not allow significant distinguishing features from non-H3K27-mutated gliomas. Ceschin et al13 evaluated serial ADC maps of patients with DIPG treated for pseudoprogression with peptide-based vaccines. They found that their cohort with pseudoprogression had significantly higher fractional decreased ADC values. Finally, Poussaint et al14 found that in a trial with DIPG that decreased ADC, increased skewness and contrast enhancement correlated with a shorter survival.
Specific low-grade gliomas such as pilomyxoid astrocytomas and pleomorphic xanthoastrocytomas (PXAs) can have worse outcomes.
Low-grade astrocytomas are the most common pediatric primary brain tumors and generally have good outcomes. When symptoms, a lack of gross total resection, and tumor growth necessitate treatment, current chemotherapy typically includes carboplatin, with a 10–20-year overall survival of 83–94%. Future therapy may include BRAF-targeting agents, as this is the most common mutation in low-grade gliomas, including pilocytic astrocytomas. However, not all low-grade gliomas have good outcomes; 6.3% have dissemination at presentation or follow-up.15 PXAs and thalamic locations are also adverse risk factors.16
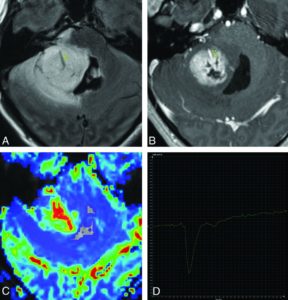
Pilomyxoid astrocytomas have been reported to have higher rates of dissemination and even malignant transformation. Our experience with DSC perfusion using 90° flip angles, which does not suppress the T1 signal, showed a high sensitivity (91%) and specificity (90%) for pilomyxoid and pilocytic astrocytomas when the percent signal return went beyond the baseline on time–signal intensity curves. However, we were not able to distinguish pilocytic and pilomyxoid pathologies using this method.17
Although rare, PXAs are outliers for low-grade gliomas in that the overall survival rates at 5 and 10 years were only 76% and 68%, respectively. Little in the literature focuses solely on PXAs. Moore et al18 described the imaging phenotype in a cohort of 9 children with PXA, showing that most are supratentorial, cortically based with inner table scalloping, and have cysts and marked adjacent edema with low ADC values and ratios compared with normal brains.