Jason Allen
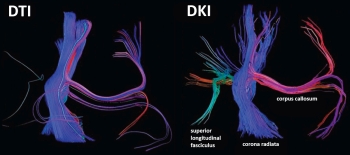
It has been apparent since the time of the early neuroanatomists that the white matter of the CNS was organized in predictable bundles. With the advent of neuroimaging, in particular, MRI, a number of these white matter tracts could be identified. However, the complexity of several of these tracts, particularly their branching and intersecting patterns, limits the utility of conventional MRI techniques. It has also been recognized that the diffusion of water molecules may be restricted by these tightly packed white matter tracts, which led to the development of diffusion tensor imaging (DTI)1 and markedly improved the imaging of the course and integrity of the major white matter tracts in both normal and disease states. With the growing adoption of DTI techniques, two important impediments have been encountered: the problem of multiple intersecting white matter tracts within a single voxel, which limits the ability to trace particular pathways in their entirety, and, on a molecular level, whether current DTI methodology accurately predicts water diffusion.
Conceptually it is easy to understand the difficulty in tracing white matter tracts when there is intravoxel fiber crossing between two or more different bundles. Multiple data-acquisition and computational models have been developed to address this problem. High angular resolution diffusion imaging (HARDI) involves the acquisition of a large number of gradient directions and, in a simplified form, all possible fiber directions are considered together during analysis with the path with the largest consistency in neighboring voxel direction identified as the most probable connection and, hence, the most likely direction for the fiber tract under consideration.2 Traditional DTI methodology tends to provide erroneous tract termination or directions when encountering complex intravoxel fiber crossing, whereas HARDI provides a more accurate representation of the underlying white matter architecture.
The development of diffusion kurtosis imaging (DKI) has provided a potentially more fundamental change in the approach to water diffusion imaging. Molecular diffusion is a random process and may be depicted by a probability distribution. If the substrate through which a water molecule is traversing is homogeneous, its distribution will have a Gaussian form, with the width of the distribution proportional to the apparent diffusion coefficient as is assumed in DTI methodology. However, water diffusion within complex biological substrates, which typically contain multiple tissue types and compartments, does not adhere to a Gaussian distribution. Kurtosis is a dimensionless metric that describes the deviation of a probability distribution from a Gaussian form and methods have been developed to estimate the kurtosis of water diffusion within the CNS.3 DKI is an extension of DTI and is acquired similarly, with the exception of the addition of higher b-values, and the same metrics of fractional anisotropy (FA) and mean diffusivity (MD) may be computed. Mean kurtosis (MK) is an additional value that may be calculated using DKI, which, again, is the measure of the degree to which water diffusion departs from a Gaussian distribution in a particular region. In the most straightforward analysis, DKI may more accurately characterize white matter tracts than DTI, possibly allowing better tracking of intersecting pathways. As MK may be conceptualized as a measure of diffusional heterogeneity of a particular region, a possible surrogate marker for the inherent complexity of a region, DKI may also provide better characterization of both gray and white matter.
The current issue of the AJNR News Digest highlights advanced MRI diffusion imaging methods, including the application of HARDI in the mapping of the complex auditory radiation pathway and the use of DKI in the investigation of diverse disease states such as Alzheimer disease (AD), multiple sclerosis, and squamous cell carcinoma as well as in the characterization of the developing brain.
In the first article, Berman et al demonstrate the superiority of HARDI to traditional DTI in the delineation of the auditory radiation, which has been previously difficult to track with imaging.4 As discussed above, traditional DTI has limited utility in situations of significant intravoxel fiber crossing or complexity. The robust anteroposteriorly aligned inferior longitudinal fasciculus (ILF) traverses the auditory radiation and DTI fiber tracking is