Human neurodevelopment is an incredibly complex and incompletely understood process, with sequential events beginning in the embryonic/fetal period and continuing throughout infancy, childhood, and adolescence. Characteristic changes at each stage of life are reflected by progression through successive neurologic and psychosocial milestones. This coordinated maturation occurs despite rapid fluctuations in growth and metabolism, highlighting the intricate interactions between genetics, epigenetics, and the environment.
This past century has seen an explosion in neuroscientific understanding, fueled by numerous advances in science, engineering, and medicine. Human embryology and animal studies have provided us with a general understanding of macrostructural processes, including landmarks for neurulation, sulcation, and myelination during fetal and postnatal life. Microstructural processes, such as neuroepithelial progenitor cell migration and differentiation in utero or synaptic connectivity and pruning from birth through young adulthood, are less well-characterized.
What has largely eluded discovery is the precise relationship between central nervous system structure and higher cognition (ie, “brain versus mind”). The developing pediatric brain contains answers to several unsolved mysteries such as neuroplasticity, neuropsychiatric disorders, and executive functioning. Current investigations into human neurodevelopment are being performed at multiple levels, ranging from molecular and cellular neurobiology to tissues and organ systems. Integration of “bottom-up” and “top-down” approaches is made possible by emerging fields such as systems biology, genomics, and clinomics.
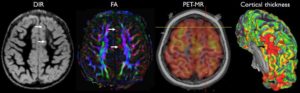
As neuroradiologists in a rapidly evolving health care landscape, we must continually provide “added value” to our clinical colleagues and patients through cutting-edge care and research. This necessitates robust translation of various neuroscientific and neurotechnological developments, particularly advances in image acquisition (diffusion, perfusion, functional, metabolic) and analysis (segmentation, morphometry, connectivity). For clinical applications, the distinction between normal variation and pathology is crucial for proper diagnosis, treatment, and follow-up. As trainees, we were taught that “normal is the hardest thing to call” and attended innumerable lectures on normal variants and pitfalls. Why, then, is normal CNS development not an essential component of neuroradiology education? For one thing, normative pediatric data are challenging to obtain, given patient referral bias and the vulnerability of the study population. Younger children are especially limited in their ability to understand and tolerate imaging examinations. Even when significant differences are reported between study and control groups, this information is not readily generalizable in light of worldwide variations in patient demographics, technical image acquisition, and analysis.
In this AJNR News Digest, we highlight several recent articles utilizing advanced imaging to investigate trends in pediatric neurodevelopment.
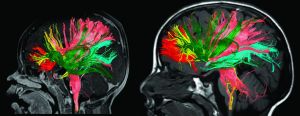
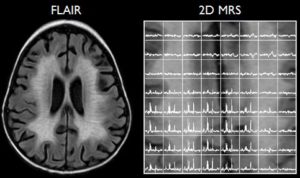
Paydar et al1 performed diffusion kurtosis imaging in patients from birth to 5 years of age. Age-related increases in fractional anisotropy were observed up to 2 years of age, while mean kurtosis was more sensitive in assessing microstructural changes in both gray and white matter, including complex multidirectional fiber patterns, beyond 2 years.
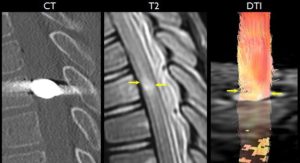
Saksena et al2 acquired diffusion tensor images of the cervical and thoracic spinal cord in healthy children between 6 and 16 years of age. This demonstrated age-, gender-, and location-related trends in fractional anisotropy, as well as mean, axial, and radial diffusivity across the cord.
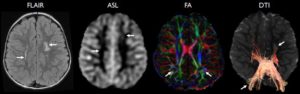
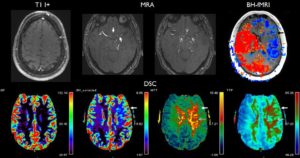
Forkert et al3 performed volumetry, arterial spin-labeling, and diffusion-weighted imaging in a cohort of pediatric patients from infancy through adolescence. Trends in increasing regional gray matter volume, decreasing apparent diffusion coefficient, and increasing cerebral blood flow were reported from 0 to 18 years of age.
Evangelou et al4 acquired 1H-MR spectroscopy in healthy pregnant women between 18 and 40 weeks of gestational age to assess in vivo metabolic maturation. Total choline remained relatively stable across gestational age, with increases in total NAA, total Cr, and their corresponding ratios.
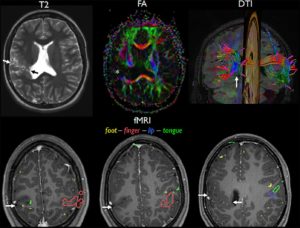
Cui et al5 utilized resting-state functional MRI (rs-fMRI) and diffusion tensor imaging to investigate the default mode network in preterm infants. Subjects with anatomic brain injury demonstrated corresponding alterations in default mode network microstructure and neurocognitive outcomes.
Li et al6 correlated rs-fMRI of the frontoparietal (central executive) networks with intelligence quotients in late childhood and adolescence. This demonstrated development of the right frontoparietal network in childhood, with later maturation of the left hemispheric network in adolescence.
Ongoing longitudinal, population-based studies include the Human Connectome Project, the Adolescent Brain Cognitive Development Study, and the Rochester Epidemiology Project. These major contributions to the growing body of reference data will spur the discovery of noninvasive imaging biomarkers across the spectrum of congenital and acquired neurologic disorders, thus enabling earlier and more accurate diagnosis, intervention, and follow-up. Advanced neuroimaging techniques—in conjunction with biochemical, genetic, and neuropsychological data—offer extraordinary opportunities to noninvasively characterize normal and abnormal neurodevelopment across the human lifespan. Continued multidisciplinary collaboration and innovation are crucial to achieve the “Holy Grail” of individualized medicine and theranostics.
References
- Paydar A, Fieremans E, Nwankwo JI, et al. Diffusional kurtosis imaging of the developing brain. AJNR Am J Neuroradiol 2014;35:808–14, 10.3174/ajnr.A3764
- Saksena S, Middleton DM, Krisa L, et al. Diffusion tensor imaging of the normal cervical and thoracic pediatric spinal cord. AJNR Am J Neuroradiol 2016;37:2150–57, 10.3174/ajnr.A4883
- Forkert ND, Li MD, Lober RM, et al. Gray matter growth is accompanied by increasing blood flow and decreasing apparent diffusion coefficient during childhood. AJNR Am J Neuroradiol 2016;37:1738–44, 10.3174/ajnr.A4772
- Evangelou IE, du Plessis AJ, Vezina G, et al. Elucidating metabolic maturation in the healthy fetal brain using 1H-MR spectroscopy. AJNR Am J Neuroradiol 2016;37:360–66, 10.3174/ajnr.A4512
- Cui J, Tymofiyeva O, Desikan R, et al. Microstructure of the default mode network in preterm infants. AJNR Am J Neuroradiol 2017;38:343–48, 10.3174/ajnr.A4997
- Li C, Tian L. Association between resting-state coactivation in the parieto-frontal network and intelligence during late childhood and adolescence. AJNR Am J Neuroradiol 2014;35:1150–56, 10.3174/ajnr.A3850
Image from: Paydar A, Fieremans E, Nwankwo JI, et al. Diffusional kurtosis imaging of the developing brain.