Our multicenter, observational study on RASopathies, was the first study to describe cranial MRI findings in infants with a RASopathy, and our starting point was an observation that the infants with Noonan syndrome had a very characteristic, steep tentorial configuration. Briefly, RASopathies are a group of rare genetic conditions with overlapping phenotypic features.1 There are over 15 genes identified in the RAS/MAPK signal pathway, and the severity of the condition varies, ranging from mild facial features to lethal malformations in accordance with the mutation type.2
Neuroimaging features in neonates with RASopathies are limited to a few case reports, and to date, no MRI study has been conducted to define the brain abnormalities in patients with a RASopathy.3-6 We, therefore, designed a case-control study to determine the occurrence of supratentorial and infratentorial abnormalities on brain MRIs in neonates with this condition; we included genetically confirmed infants with severe clinical symptoms requiring admission to 3 neonatal intensive care units.
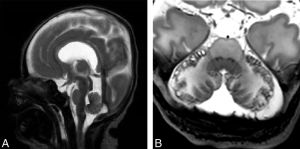
We reviewed brain MR studies of 16 patients with RASopathies and revealed that these infants had characteristic, acquired, and structural abnormalities in the posterior fossa, including peripheral cerebellar hemorrhage, vermis hypoplasia, and a steep tentorial configuration when compared with healthy controls.
These infants also showed an increased incidence of cerebral white matter lesions, enlarged extracerebral spaces, simplification of the cortical folding, and structural corpus callosum abnormalities involving the splenium.
Our hypothesis is that these structural and acquired changes can be explained as the effects of a genetic disorder, causing disruption of developmental processes, which results in a permanent change in the morphology of the cranium and its components.