Amir Paydar
Human embryology and development is indeed one of the most (if not the most) fascinating subjects in the field of biological sciences. Nevertheless, our scientific community still faces challenges in trying to understand the concepts that define the underlying mechanisms of embryology and tissue development. After all, it is a very complex subject to grasp and many of the processes that take place from zygote to adulthood are yet to be ascertained. Despite this challenge, we have come to recognize that understanding the natural course of normal tissue development on both microscopic and macroscopic scales is the key to deciphering the mechanisms that take place during tissue injury and degeneration as well as healing and scarring.
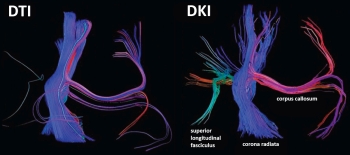
Realizing this concept, my good friends and colleagues at New York University (NYU) Medical Center decided to take on an ambitious human study to investigate brain maturation using noninvasive imaging techniques in the pediatric population. Our research subjects included 59 healthy infants with an age spectrum ranging from birth to approximately 5 years of age, when the postnatal brain is in its most active stage of development. We implemented an innovative MRI diffusion technique called diffusional kurtosis imaging (DKI) to study the microstructural changes that occur in both the WM and GM in the developing brain.
Macrostructural changes that take place within the brain during the course of maturation have been well documented by conventional MRI techniques in both normal and pathologic states. However, these conventional techniques are limited in their ability to quantify developmental changes that occur at the microstructural level. Diffusion imaging, particularly the widely used diffusion tensor imaging (DTI), has been shown to be sensitive to age-related microstructural changes that occur within the network of WM tracks as a result of myelination. However, DTI is based on