In the rapidly evolving landscape of postsurgical, postradiation therapeutic strategies for patients with glioblastoma (GBM), including cytotoxic chemotherapy, antiangiogenic therapy, and immune therapy, bevacizumab has been established as a primary therapeutic option for recurrent GBM1,2 and has also shown substantial promise in the setting of newly diagnosed GBM.1,3 As a recombinant humanized monoclonal antibody, bevacizumab blocks angiogenesis by inhibiting vascular endothelial growth factor, thereby decreasing tumor neovascularity and stabilizing the BBB.
Although bevacizumab is associated with a significant radiographic response rate and improved 6-month progression-free survival (PFS) compared with standard therapy in patients with both recurrent and newly diagnosed GBM, it has not shown a definitive improvement in overall survival (OS).4 However, more recent investigations have suggested that the addition of bevacizumab may prolong OS in a subset of patients with newly diagnosed GBM.5 Given these improved outcomes, bevacizumab will likely remain a mainstay in the treatment of patients with GBM. However, its use is tempered by high costs and potential serious adverse effects such as hemorrhage, thromboembolic events, and proteinuria.4
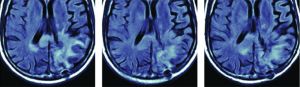
Due to its powerful antiangiogenic effects, bevacizumab results in a marked decrease in contrast enhancement, edema, and mass effect on MR imaging, which can be seen as early as a few days to weeks after starting bevacizumab.6 Despite this impressive radiographic response, there is often persistent tumor histopathologically that manifests as areas of nonenhancing hyperintense signal on FLAIR images. This phenomenon is referred to as pseudoresponse.
In the setting of pseudoresponse, conventional imaging becomes unreliable in determining persistent/recurrent tumor versus true treatment response. As a result, advanced imaging methods including diffusion, perfusion, and spectroscopy become essential in order to accurately monitor these patients. Because the ADC derived from DWI is known to correlate with tumor cellularity, DWI is a powerful technique for the detection of residual or recurrent tumor in the setting of bevacizumab therapy. For instance, one of the articles highlighted in this edition states that pretreatment ADC histogram analysis can predict survival in patients with recurrent GBM treated with bevacizumab, with lower ADC values associated with worse PFS and OS.7 Another featured article discusses the use of DSC MR perfusion in assessing treatment response in patients with recurrent GBM treated with superselective intra-arterial cerebral infusion of bevacizumab.8 Although standard contrast-enhanced imaging is limited in evaluating treatment response after bevacizumab, a recent study, which is also highlighted here, shows that delayed contrast-enhanced MR imaging can better predict treatment response.9