Guest Editor
Julie Bykowski, MD
The multisystem involvement of IgG4-related disease (IgG4-RD) has been identified only within the past 15 years,1,2 over a century since Mikulicz’s report3 of a man with swelling of the lacrimal, submandibular, and parotid glands. Pancreatitis and sialadenitis are the 2 most common manifestations of IgG4-RD; however infiltration and fibrosis/sclerosis have been reported in nearly all organ systems, and multisystem involvement may occur over time.4 It is important to be alert to the possibility of renal involvement by IgG4 when protocoling contrast-enhanced imaging exams.
The underlying etiology is not known. Histopathologic features include IgG4 plasma cell and CD4+ T lymphocyte infiltration, with tissue fibrosis or sclerosis and obliterative phlebitis.5,6 Authors suggest that the clinical manifestations are responsive rather than a primary pathology such as an imbalance in the regulatory immune reaction.7 The differential diagnosis in the setting of multifocal involvement includes Sjögren syndrome, sarcoidosis, multicentric Castleman disease, granulomatosis with polyangiitis (Wegener), and lymphoma. Diagnostic criteria vary somewhat,6,8 however, and include: clinical manifestations of IgG4-RD, elevated serum IgG4 (>135 mg/dl), and elevated ratio of IgG4+/IgG+ plasma cells (>40–50%, usually <6%) or elevated IgG4+ cells/high power field of biopsy sample in any affected organ. It is important to note that IgG4+ cell counts are also elevated in other disease processes, such as sarcoidosis, and patients may have normal serum IgG4 concentrations even when classic histopathologic changes are present in tissue samples. Fine-needle aspiration is not sufficient for accurate cell counts.
Recent AJNR articles have identified relevant imaging findings in IgG4-RD to help refine the differential diagnosis:
Extraocular muscles: Often bilateral and spares tendons (unlike pseudotumor),9 involves lateral rectus earlier (unlike Graves ophthalmopathy). Remember that most cases of inflammatory pseudotumor are not part of the IgG4 spectrum.
Orbital fat: May see signal changes associated with infiltration9; distinct extraconal or intraconal T2-hypointense, enhancing masses.11
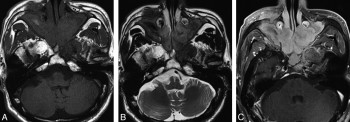
Lacrimal glands: Often bilateral, enlargement without distortion of the globe9; may see T2-hypointense, homogeneously enhancing mass10; visible vascular flow voids within the lacrimal glands favors IgG4 over lymphoma.
Salivary glands: Often multifocal, diffuse gland enlargement with homogeneous enhancement10; may see distinct T2-hypointense mass.
Cranial nerves: Pseudotumor,11 which can extend to the cavernous sinus and Meckel cave.9,10
Pituitary: T2-hypointense mass and enlargement of both the anterior and posterior lobes as well as the pituitary stalk (infundibulohypophysitis).10
Meninges: T2-hypointense, enhancing dural thickening (sclerosing pachymeningitis).10
Sinuses: IgG4-RD mimics lymphoma and may also have bone and perineural involvement.12 Concomitant sinusitis is more common in the orbital IgG4-RD than in lymphoma13 or Sjögren syndrome.6