Over the past 3 decades, many pathologic and imaging studies1–4 have supported the notion that gray matter (GM) involvement is a critical feature in the progression of MS. Importantly, GM damage has been shown to be clinically relevant because it helps to explain some of the clinical manifestations of MS, including cognitive impairment, depression, and fatigue,5 and is also a predictor of the long-term evolution of the disease.6
Different pathologic features have been seen in the GM of patients with MS. Focal lesions have been shown to involve the cortex and deep GM nuclei, as well as the cerebellar cortex and GM of the spinal cord. Cortical lesions (CLs) have been classified according to their location in the cortex with respect to the white matter (WM) interface and pial surface.7 Extensive subpial demyelination has been proposed as the distinctive hallmark of MS.8 GM pathology also includes neuronal injury, synaptic and dendritic abnormalities, and Wallerian and transsynaptic degeneration.9,10
Consistent with pathologic observations, many MRI studies have shown a marked involvement of the GM in MS, with focal lesions, “diffuse” tissue abnormalities, and irreversible tissue loss (ie, atrophy). Despite the significant efforts made to improve characterization of the type and extent of GM damage in patients with MS, most GM lesions remain undetected by current MR techniques, and there is an urgent need to develop new MR sequences and postprocessing procedures capable of accurately quantifying GM involvement, as highlighted by the papers selected for this Digest.
A postmortem MRI and histopathology study of 27 formalin-fixed coronal hemispheric brain sections from 15 patients with MS11 showed that, at 7T, T2-weighted and T2*-weighted sequences have a similar sensitivity in detecting CLs, allowing the retrospective identification of more than 80% of CLs. However, without histopathologic information, 84% of CLs still went undetected when assessed prospectively.
Pareto et al12 investigated the influence of juxtacortical lesions, detected using the FLAIR sequence at 3T, on cortical atrophy and local cortical thinning in a large sample of 131 patients at the early stages of MS (ie, clinically isolated syndrome or relapsing-remitting MS). The presence of juxtacortical lesions correlated with cortical thinning and subcortical GM atrophy in these patients. However, no anatomic correspondence was found between areas of cortical thinning and regions with an increased number of juxtacortical lesions. Such a discrepancy could be interpreted as the result of undetected CLs.
Perfusion MRI can identify GM abnormalities even in the absence of visible focal lesions on routine structural imaging. Using this approach, Hojjat et al13 quantified perfusion abnormalities in normal-appearing (NA) and lesional tissue in patients at different relapsing-remitting MS stages. Perfusion reduced with disease progression, and this effect was greater in NAGM and NAWM compared with lesional areas. Interestingly, perfusion abnormalities in the NAGM but not those of the NAWM helped to distinguish patients with MS and cognitive impairment from healthy controls.
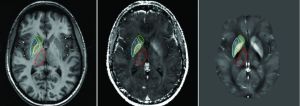
Because GM abnormalities in MS can also result from retrograde or anterograde Wallerian degeneration, another investigation used the Network Modification Tool (which infers structural connectivity loss from WM abnormalities) to assess the relationship between GM atrophy and abnormalities in connecting WM in a large group of patients with early stages of MS.14 A significant correlation was found between atrophy of the deep GM nuclei (thalami and putamen) and abnormalities in connecting WM, suggesting an increased susceptibility to degenerative phenomena of