Dementia is an epidemic affecting over 35 million people worldwide, costing over $600 billion annually.1 Given the increasing prevalence and economic impact of mild cognitive impairment (MCI) and dementia, there have been intensified efforts in early identification and early intervention to improve treatment success.1 Neuroimaging can play a central role in the early diagnosis of dementia, but there are challenges with standard neuroimaging, including overlapping imaging findings with other commonly seen findings in the aging population and lack of widespread availability. Both high-resolution MRI and newer forms of molecular imaging can be used to assess and diagnose dementia.
After other potentially treatable findings such as masses or intracranial hemorrhage have been excluded, standard neuroimaging with CT or MRI can be used for more detailed assessments of global parenchymal volume and region- and lobar-specific volumes. Both qualitative and quantitative assessments of patterns of volume loss, often with standard or volumetric T1-weighted MR images, are helpful in differentiating types of dementia.2 Assessing volume in specific regions, including the hippocampus, entorhinal cortex, cingulate gyrus, and precuneus, is important in the early identification of many dementias, especially Alzheimer disease.3-5 If quantitative evaluation is not available, many quick and reproducible visual rating systems have been described to assess degrees of atrophy.6-8 One of the highlighted studies provides new potential imaging biomarkers for dementia and MCI on MRI.9 In addition to standard sequences, there is evidence of alterations in hemodynamics in brain regions affected in certain types of dementia, which may also be helpful in diagnosing and differentiating dementia types.10
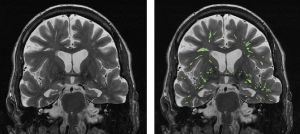
There are many other imaging findings seen on routine imaging associated with dementia and MCI. There is strong evidence that markers of cerebral small vessel disease, specifically white matter hyperintensities, covert brain infarctions, and cerebral microbleeds, are associated with cognitive dysfunction.11-15 While these findings are strongly associated with cognitive impairment, they are also commonly encountered in older patient populations, which limits diagnostic certainty. Another commonly encountered finding that may be associated with MCI is increased density of enlarged perivascular spaces, as described in one of the highlighted articles.16
Beyond standard neuroimaging, there are several advanced imaging techniques that are helpful in diagnosing dementias. FDG-PET, which evaluates for spatial patterns of hypometabolism, is one of the most commonly used advanced imaging techniques in clinical practice.17 Recent advances including amyloid PET and tau PET imaging also show significant promise. One of our highlighted articles explores the association between β-amyloid, brain atrophy, and cognitive decline.18
Newer methods of imaging that may provide insight into the pathophysiology underpinning the development of dementia include imaging of the glymphatic system. The glymphatic system is a brain waste clearance pathway which drains soluble waste proteins and metabolic byproducts and is thought to play a role in dementia, brain aging, and other pathologic processes. The role of imaging the glymphatic system is evolving, with recent contributions from one of our highlighted articles.19
In this installment of the AJNR News Digest, we highlight several recently published articles that address potential imaging findings in the setting of dementia and mild cognitive impairment and also future directions in imaging. With a constantly evolving role, neuroimaging is critical in the early identification of dementia subtypes.