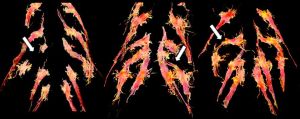It is my pleasure to write an editorial for this exciting AJNR News Digest on the topic of MR neurography (MRN). MRN has come a long way since its introduction in 1992 by Howe et al.1 Several technical developments have occurred in this domain over the last decade,2–6 and the increasing use of MRN has impacted and considerably changed the practice of neurology, neurosurgery, and plastic surgery as it pertains to the diagnostic evaluation and treatment of peripheral neuropathy and plexopathy.7–11
High-resolution isotropic 3D imaging with thick-slab isotropic MIPs delineates the brachial plexus nerves in multiple arbitrary planes, as shown by Vargas et al12 and our group.13 3D variable turbo factor spin-echo imaging partially mitigates vascular signal contamination in the vicinity of the nerves. Near-complete vascular signal suppression for MRN is successfully achieved using 1 of these methods: reversed fast imaging with steady-state free precession sequence, high-b-value diffusion imaging, and addition of motion sensitive driven equilibrium to 3D Dixon or 3D spectral-attenuated inversion recovery imaging.14 Such nerve-selective images reconstructed along the long axis of the nerve lead to increased conspicuity of nerve abnormalities, such as intraneural signal and fascicular and/or caliber alterations.15 The extents of the neuroma and nerve gap are also well-delineated and characterized. The accuracy of MRN for both small and large nerves has been well-established in multiple scientific studies.16–19
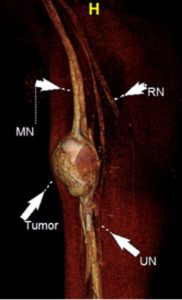
Lumbosacral (LS) plexus anatomy is complicated, and the branch nerves course obliquely from their origins to innervate the extremities. Cho Sims et al20 recently showed that 3D isotropic vascular and fat signal-suppressed imaging selectively demonstrates LS plexus anatomy when the images are reconstructed in specific angular planes in sagittal and coronal orientations. Plexus and peripheral nerves can be affected by several diffuse conditions, such as acute and chronic demyelinating conditions, autoimmune neuropathy, hereditary motor and sensory neuropathy, vasculitis, and lymphoma. Thawait et al21 and others22 have highlighted the MRN imaging appearances of diffuse polyneuropathy conditions with excellent illustrations.
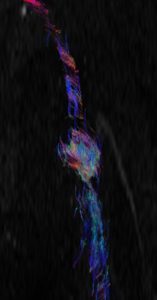
DTI allows for interrogation into the internal architecture of the peripheral nerves and provides quantitative information in the form of fractional anisotropy (FA) and ADC. Higher-signal imaging on newer 1.5T and 3T scanners has made DTI feasible for peripheral nerves. Decreasing FA and increasing ADC has been shown to correlate with nerve degeneration, while increasing FA and decreasing ADC has been shown to correlate with nerve regeneration. DTI is being employed in both clinical and research settings as a functional part of MRN for the evaluation of nerve entrapment, diffuse neuropathy, and nerve tumor, and for treatment response following nerve surgery. Incremental diagnostic value of DTI has been shown when it is combined with anatomic MRN,23–26 and it can aid in the diagnosis of neuropathies and the presurgical planning for peripheral nerve sheath tumors.
This Digest includes exciting and innovative works by Dr. Fujii on 3D peripheral nerve visualization, Dr. Fayad on MR spectroscopy of nerve sheath tumors, and Dr. Eguchi, Dr. Ahlawat, and Dr. Yuh on the novel use of DTI in neuropathy and peripheral nerve sheath tumors.
The future of MRN is extremely bright given the demand from various specialists and its unique advantages of being able to provide an anatomic neuromuscular picture noninvasively and to offer high sensitivity for finding and localizing a neural abnormality. I foresee an increasingly significant role for MRN and its widespread use in the diagnostic realm of peripheral neuropathy, decision-making (conservative versus surgical management), planning of image-guided injections and surgery, intraoperative guidance, and prognosis. Patient outcome studies8,9 are being published and will likely establish the cost-effectiveness of this novel technology.
References
- Howe FA, Filler AG, Bell BA, et al. Magnetic resonance neurography. Magn Reson Med 1992;28:328–38, 10.1002/mrm.1910280215
- Zhang ZW, Song LJ, Meng QF, et al. High-resolution diffusion-weighted MR imaging of the human lumbosacral plexus and its branches based on a steady-state free precession imaging technique at 3T. AJNR Am J Neuroradiol 2008;29:1092–94, 10.3174/ajnr.A0994
- Qin Y, Zhang J, Li P, et al. 3D double-echo steady-state with water excitation MR imaging of the intraparotid facial nerve at 1.5T: a pilot study. AJNR Am J Neuroradiol 2011;32:1167–72, 10.3174/ajnr.A2480
- Chhabra A, Subhawong TK, Bizzell C, et al. 3T MR neurography using three-dimensional diffusion-weighted PSIF: technical issues and advantages. Skeletal Radiol 2011;40:1355–60, 10.1007/s00256-011-1162-y
- Fujii H, Fujita A, Yang A, et al. Visualization of the peripheral branches of the mandibular division of the trigeminal nerve on 3D double-echo steady-state with water excitation sequence. AJNR Am J Neuroradiol 2015;36:1333–37, 10.3174/ajnr.A4288
- Manoliu A, Ho M, Nanz D, et al. MR neurographic orthopantomogram: Ultrashort echo-time imaging of mandibular bone and teeth complemented with high-resolution morphological and functional MR neurography. J Magn Reson Imaging 2016;44:393–400, 10.1002/jmri.25178
- Andreisek G, Burg D, Studer A, et al. Upper extremity peripheral neuropathies: role and impact of MR imaging on patient management. Eur Radiol 2008;18:1953–61, 10.1007/s00330-008-0940-y
- Petrasic JR, Chhabra A, Scott KM. Impact of MR neurography in patients with chronic cauda equina syndrome presenting as chronic pelvic pain and dysfunction. AJNR Am J Neuroradiol 2017;38:418–22, 10.3174/ajnr.A4994
- Fisher S, Wadhwa V, Manthuruthil C, et al. Clinical impact of magnetic resonance neurography in patients with brachial plexus neuropathies. Br J Radiol 2016;89:10.1259/bjr.20160503
- Cox B, Zuniga JR, Panchal N, et al. Magnetic resonance neurography in the management of peripheral trigeminal neuropathy: experience in a tertiary care centre. Eur Radiol 2016;26:3392–400, 10.1007/s00330-015-4182-5
- Chhabra A, Belzberg AJ, Rosson GD, et al. Impact of high resolution 3 Tesla MR neurography (MRN) on diagnostic thinking and therapeutic patient management. Eur Radiol 2016;26:1235–44, 10.1007/s00330-015-3958-y
- Vargas MI, Viallon M, Nguyen D, et al. New approaches in imaging of the brachial plexus. Eur J Radiol 2010;74:403–10, 10.1016/j.ejrad.2010.01.024
- Chhabra A, Thawait GK, Soldatos T, et al. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol 2013;34:486–97, 10.3174/ajnr.A3287
- Wang X, Harrison C, Mariappan YK, et al. MR neurography of brachial plexus at 3.0 T with robust fat and blood suppression. Radiology 2017;283:538–46, 10.1148/radiol.2016152842
- Baumer P, Kele H, Kretschmer T, et al. Thoracic outlet syndrome in 3T MR neurography—fibrous bands causing discernible lesions of the lower brachial plexus. Eur Radiol 2014;24:756–61, 10.1007/s00330-013-3060-2