Lea Alhilali
Gliomas constitute the most common primary brain neoplasms in adults and comprise a diverse spectrum of tumors, histologically ranging from low-grade, indolent neoplasms to high-grade, aggressive lesions. Accurate, prospective grading of primary cerebral gliomas is critical for therapeutic planning and determining prognosis. Unfortunately, histopathologic grading of these lesions, either by stereotactic biopsy or surgery, is not without error; stereotactic biopsy is subject to sampling errors, and surgical tissue sampling cannot assess regions of residual tumor that were not removed during cytoreductive surgery.
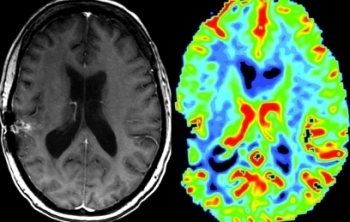
Unlike surgical methods, MR imaging is not inherently limited by incomplete sampling and has the benefit of imaging the entire lesion. Conventional MR imaging does provide valuable information on several features suggestive of a high-grade, aggressive lesion, including breakdown of the blood-brain barrier and enhancement, central necrosis, hemorrhage, mass effect, and satellite lesions. Unfortunately, there can be significant overlap in the imaging appearance of the different glioma grades, with high-grade lesions occasionally lacking enhancement, prominent mass effect, or necrosis, and appearing similar to low-grade lesions. Similarly, low-grade lesions can sometimes demonstrate aggressive features, including enhancement, necrosis, or hemorrhage. Furthermore, conventional MR imaging does not provide reliable information on physiologic parameters central to tumor grading, including angiogenesis, metabolism, and microvascularity.
Unlike conventional MRI, perfusion MRI is not dependent on breakdown of the blood-brain barrier and can demonstrate important features essential in tumor grading, including microvascularity and angiogenesis. In this issue of the AJNR News Digest, we focus on the work of authors whose research has advanced the use of MR perfusion in the grading of primary gliomas.
Dynamic susceptibility contrast (DSC) perfusion imaging was the first perfusion imaging technique to show a reliable correlation between the relative cerebral blood volume (rCBV), tumor grade, and increased tumor vascularity on histology. However, reproducibility of rCBV measurements has been a concern, which is an issue of increasing importance, given