Prof. François Nicoli
“There are three principal cranial collateral pathways…. (including) pial anastomoses between and among the three major cerebral arteries. These collateral pathways exist awaiting demand, and enlarge as demand for flow rate and volume increases. It is our impression that (stroke) prognosis is related to the richness of demonstrable pathways.”
These sentences are quite topical, but were formulated in 1966 by Love et al,1 in their paper on cranial collateral pathways and stroke, based on conventional angiography and published in the American Journal or Roentgenology. Fifty years later, thanks to the development of neuroimaging techniques and acute stroke treatments, many studies have provided evidence supporting the impression of Love et al.
As a young physician early involved in the field of neurology and also working in the neuroradiology department of Professor Georges Salamon at La Timone University Hospital in Marseille in 1984, my awareness was raised about the importance of collateral circulation in patients with ischemic stroke. Choosing later to focus my clinical work on cerebrovascular diseases, I was particularly interested by papers about collateral circulation and notably one formulating an exciting hypothesis about the possible synergy of collateral flow and intravenous thrombolysis to recanalize MCA M1 occlusion. Indeed, in 2005, Liebeskind2 suggested that “the better the collateral flow is, the higher the recanalization rate after intravenous thrombolysis (IVT) should be …. thanks to a retrograde delivery of rtPA to the distal end of the clot.” Actually, as early as 1992, Ringelstein et al3 also proposed the same assumption about the possible impact of collateral flow on recanalization. In addition, in 1993, von Kummer and Forsting4 provided preliminary data in agreement with the hypothesis of Ringelstein. Indeed, in a small cohort of 29 patients with MCA M1 occlusion treated with intravenous or intra-arterial rtPA within 6 hours after symptom onset and invasively explored using conventional angiography before and after thrombolysis, a single univariate analysis suggested that partial or complete MCA M1 recanalization was significantly more frequent after thrombolysis in patients with good collateralization on baseline angiography.4
The Quest for a Noninvasive Assessment of the Vigor of the Collateral Flow
The aim of my study was specifically to noninvasively test the hypothesis formulated by Ringelstein and Liebeskind.2,3,5
To achieve this goal, a retrospective analysis was performed over 64 patients with an acute MCA M1 occlusion, treated at the comprehensive stroke center of La Timone University Hospital (Marseille, France) and recorded into a prospective clinical registry for IVT. Thanks to repeated regional public awareness campaigns on stroke warning signs and an optimized acute stroke pathway using medical advanced regulation for stroke, these patients were consecutively included in the prospective registry within a short period of time (from January 2009 to December 2010), which guaranteed very homogeneous medical and radiologic management.6 We defined an index derived from the baseline MR perfusion imaging in order to noninvasively and indirectly quantify the collateral circulation deficit. It was decided to analyze MCA M1 recanalization 24 hours after IVT because a full MCA recanalization within 24 hours post-IVT is a strong predictor of good stroke outcome at 3 months.7 Another reason is that, in our stroke imaging protocol, all patients systematically had a multimodal MRI at the hyperacute phase of stroke, and a CT angiography 24 hours after IVT.5
Briefly, using a new PWI-derived collateral flow index based on Tmax maps at different time points, this work provided the first evidence that a good baseline supplying flow significantly increases the rate of full recanalization at 24 hours in patients with acute MCA M1 occlusion treated with IVT within 3 hours after stroke onset.5 A similar conclusion was later made by other authors using different methods.8–10
Thus, a better collateral flow is associated with a higher recanalization rate not only after endovascular therapy11 but also after systemic thrombolysis. Indeed, the positive impact of the vigor of the collateral flow on full MCA recanalization is obvious in patients treated by an endovascular route using the Merci retriever (Concentric Medical, Mountain View, California).11,12 However, it becomes negligible in patients treated with a stent retriever thanks to a much higher efficacy on recanalization of stent retrievers compared with the Merci retriever.13 Nevertheless, whatever the type of retriever considered, the vigor of the collateral flow is an independent predictor of clinical outcome after endovascular thrombectomy12,13 and remains a major predictor of ischemic stroke outcome.
Our study also demonstrated that the probability of full MCA M1 recanalization at 24 hours significantly decreases in patients treated with IVT beyond 3 hours after stroke onset. More recently, Muchada et al14 found a similar time dependency of thrombolysis-induced recanalization in patients with proximal MCA occlusion. Hopefully, the recent great success of IVT coupled with the use of stent retrievers counterbalances this unexpected limitation of IVT performed alone.13
Noninvasive Quantification of the Arterial-to-Tissue Delay and Mapping of the Collateral Flow Deficit
The PWI-derived collateral flow index herein described and named normalized collateral circulation decifit index (nCCD) was useful to noninvasively assess the vigor of the collateral blood flow. However, it cannot be automatically computed and does not provide a map of the collateral flow deficit. Thus, the next step of this research was to test, on the same clinical and radiologic database (PWI), a new Bayesian algorithm able to accurately quantify the arterial-to-tissue delay (ATD), physiologically correlated to the vigor of the collateral flow in case of intracranial and/or cervical arterial occlusion.12,15
A preliminary analysis demonstrated that the volume of tissue with an ATD higher than 6 seconds (termed VolATD6) had the best correlation with the nCCD index (linear regression, r2 = 0.72, P <.0001).15 In addition, nCCD and VolATD6 are significantly correlated to the relative speed of the retrograde perfusion (r2 = 0.45, P = .0001; r2 = 0.56, P <.0001, respectively).15,16 As expected, like nCCD, VolATD6 is also predictive of full MCA M1 recanalization after IVT.16,17 Using a similar method based on an ATD measurement, Zhang et al18 very recently confirmed these results. Furthermore, this strategy allowed a highly reproducible automatic measurement and mapping of tissue with increased ATD, which is more suitable for emergency radiologic management of patients with hyperacute stroke.15
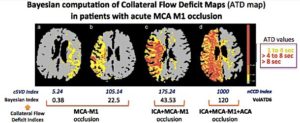
Two patients had a proximal MCA M1 occlusion, one a tandem occlusion and the other a terminal ICA-T occlusion. For each patient, nCCD and VolATD6 values were calculated. nCCD is a cSVD index based on Tmax maps at different time points and calculated using a block-circulant Singular Value Decomposition algorithm (cSVD). VolATD6 is a Bayesian index based on arterial-to-tissue delay maps calculated using a Bayesian algorithm.
Correlations with the Angiographic Collateral Grading
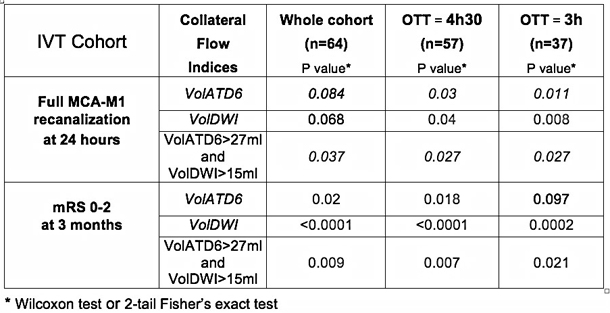
Thanks to a fruitful scientific collaboration with the UCLA Stroke Investigators, it was possible to demonstrate, in a cohort of patients with acute MCA M1 occlusion treated by an endovascular route (mostly with a Merci retriever) and explored using MR PWI before angiography, that VolATD6 is significantly correlated with the angiographic collateral grading.12,15 This angiographic classification of the collateral flow was evaluated with the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) Collateral Flow Grading System on baseline angiography. Using this angiographic scale, patients are assigned to Grade 0 (no collaterals visible to the ischemic site), 1 (slow collaterals to the periphery of the ischemic site with persistence of some of the defect), 2 (rapid collaterals to the periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory), 3 (collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase), and 4 (complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion). A receiver operating characteristic analysis determined that the optimal threshold of prolonged ATD tissue volume providing the best discrimination between angiographic grades 0–2 versus 3–4 was: VolATD6 threshold=27 mL (AUC = 0.84; sensitivity = 100 %; specificity = 67%; Youden index = 0.67; P <.0001).12 Interestingly, in this cohort, a pretreatment DWI lesion volume (VolDWI) value of 15 ml also provided a clear-cut discrimination between angiographic grades 0–2 versus 3–4 (sensitivity = 80 %; specificity = 85.7 %; Youden index = 0.66; P <.0001)12
Predictive Value of Collateral Flow Indices on MCA M1 Recanalization and Clinical Outcome after Intravenous Thrombolysis
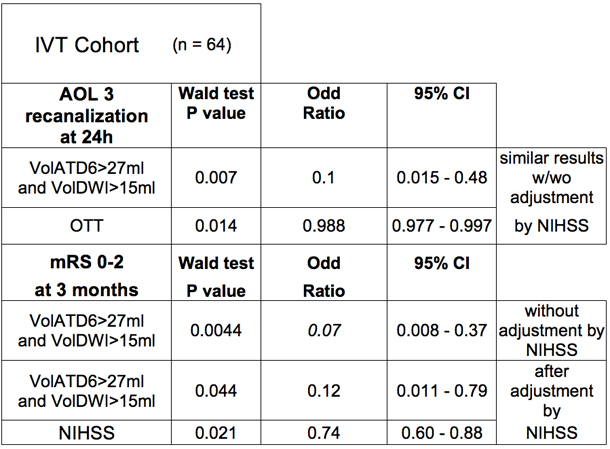
This final step was to evaluate, in the initial IVT patient cohort, the predictive value of these new parameters assessing the vigor of the collateral flow, on full MCA M1 recanalization 24 hours after IVT.17 In this cohort, a VolATD6 value lower than 20 mL, corresponding to an excellent collateral flow,12 predicts full MCA M1 recanalization 24 hours after IVT in 90% of cases.17 Interestingly, at the time of the MRI examination, the VolATD6 measurement provides an instantaneous estimation of the degree of collateral flow while the VolDWI measurement corresponds to a tissue marker for the efficiency of collateral perfusion to preserve tissue from ischemia during the time to MRI.