Hunt MA, Bagó AG, Neuwelt E. Single-Dose Contrast Agent for Intraoperative MR Imaging of Intrinsic Brain Tumors by Using Ferumoxtran-10. AJNR Am J Neuroradiol 2005;26:1084–88
 Matthew A. Hunt |
 Daniel Guillaume |
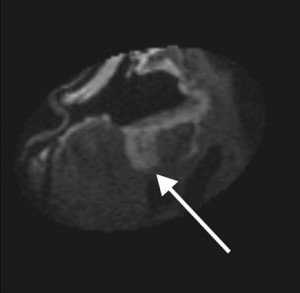
Intraoperative MRI during surgical resection of brain tumors has been developed to improve the extent of resection of glioblastoma and other malignant brain tumors in adults and children. The technique provides nearly immediate feedback as to whether the intended surgical goals have been attained (Figure 1). Early on, the risks of repeat administration of gadolinium-based contrast agents (GBCA) were recognized as a potential pitfall of intraoperative MRI. This problem resulted mainly from surgically induced disruption of the BBB or alterations in the regional perfusion around the surgical cavity. Ultrasmall paramagnetic iron oxide (USPIO) nanoparticle imaging emerged as a potential solution to this problem, given its unique properties. Rather than rapidly crossing the leaky BBB and rapidly exiting, USPIO nanoparticles, and particularly ferumoxtran-10, which was used in this report, have a long plasma half-life, cross the BBB slowly, and then are trapped after being taken up by tumor-associated inflammatory cells.
This paper showed that the early-generation USPIO ferumoxtran-10 provides a way to decrease non-tumor-related enhancement during intraoperative MRI from surgically induced BBB injury or vascular changes when compared to GBCA. Subsequently, ferumoxytol has become the preferred USPIO imaging agent due to advantages in administration as well as an FDA-approved indication for iron replacement. This agent has similar imaging properties to ferumoxtran-10 and the ability to be administered as a bolus, which has also allowed for dynamic and vascular blood pool imaging studies.
Figure 1. Intraoperative postresection T1WI in a patient with glioblastoma multiforme receiving ferumoxtran-10 for intraoperative imaging, demonstrating residual enhancing lesion posteriorly (arrow). This lesion was then localized by using integrated frameless stereotaxy and resected. Reproduced from Hunt et al, AJNR 2005.
Clinical studies also used the early intravascular characteristic of the USPIOs to measure blood volume in intracerebral lesions using perfusion MRI.1,2 Building on our preclinical and clinical findings, a clinical study