Over one-third of people with mild cognitive impairment (MCI) progress to Alzheimer disease (AD) in 5 years or longer. These findings have naturally generated an interest in using disease-modifying agents to not only preserve cognitive function in individuals with MCI but also slow their conversion to dementia.1 However, the early detection of MCI remains one of the biggest clinical challenges. Several tools, such as the Mini Mental State Exam, are used by physicians to clinically assess for MCI. Failure to detect this condition, especially in its early stages, remains a significant limitation of these tools and underscores an emergent need for more quantitative and sensitive diagnostic approaches.
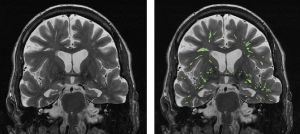
Perivascular spaces (PVSs) are cavities filled with cerebrospinal and interstitial fluids that lie between the perforating blood vessels of the brain and the pia mater. They act as conduits for the drainage of interstitial fluid and solutes from the brain.2 The measurement of enlarged PVSs reflects brain functionality that may be independent from brain tissue atrophy, the biomarker targeted with conventional MRI methods. Hence, we hypothesized that enlarged PVSs may be an earlier signature than structural brain atrophy for the diagnosis of MCI.
There are difficulties for PVS MRI due to the fine and sporadic structures of PVSs. Current evaluation of their properties is mostly limited to the subjective and manual assessment of their shape, size, and numbers. We therefore aimed to develop an objective and quantitative MRI method for the automatic segmentation and mapping of enlarged PVSs in the brain. With the datasets kindly provided by Drs. Das and Yushkevich from the University of Pennsylvania, we developed an innovative PVS segmentation algorithm relying on the high spatial gradient of PVSs in T2-weighted brain MR images, generating 3D views of high-resolution PVS maps. In addition, we were able to quantify PVS density—the percentage of PVS over the total brain volume—as a quantitative biomarker for diagnosing MCI pathology. ROC analysis demonstrated over 90% of sensitivity and specificity for the differentiation between patients with MCI and healthy controls based on the quantified PVS density.