It is my pleasure to serve as the guest editor of this edition of the AJNR News Digest, which focuses on the topic of image-guided spine pain management. Disorders of the spine have a tremendous impact on society—directly through the morbidity of afflicted individuals and indirectly through lost productivity and increased health care costs. In 1998, total U.S. health care expenditures for low back pain were estimated at $90 billion.1 Since that time, costs of low back and neck pain care have risen substantially, at a rate higher than that observed for overall health expenditures.2 In the United States, an estimated 149 million work days are lost every year because of low back pain.3
Spinal injections of steroids have been widely used in the management of back pain for more than 50 years.4 Image-guided spine injections (when performed for appropriate indications) and using contemporary techniques can provide significant benefits in relation to pain relief, disability, and quality of life in these patients, identify the specific pain generator, and help avoid surgery. These injections have a low-risk profile compared with alternative treatments and are effective when used as part of a multimodal treatment plan that includes physical therapy, exercise, and activity modifications.
Between 1994 and 2001, the use of epidural steroid injections increased by 271% and facet joint injections by 231% among Medicare beneficiaries.5 Medicare payments for spinal injections expanded 629% during that time period.5 More recent data indicate continued rapid growth in the use of spinal injection therapies. The success of spinal injections in alleviating symptoms depends on the ability to deliver the anti-inflammatory medication (corticosteroid) precisely to the specific site of inflammation. For example, with disc herniations, the inflammatory response (and hence the site of pain generation) is at the interface of the herniated disc and traversing neural tissue. This site is frequently within the ventral epidural space, subarticular zone, or neural foramen. For this reason, transforaminal epidural steroid injections (TFESIs) may be more effective than interlaminar epidural steroid injections (ILESIs) in targeting the specific pain generator in the setting of an acute disc herniation.
Image-guided spinal injections have a high safety profile; however, appropriate risk mitigation techniques are necessary to avoid extremely rare, catastrophic complications, such as direct spinal cord or vascular injury or CNS infarcts.6 Risk mitigation techniques include thoroughly reviewing preprocedure imaging (to identify the pain generator and vulnerable soft tissue structures), using appropriate imaging techniques during the procedure (eg, DSA or CT fluoroscopy for vascular uptake), performing a contrast test injection, choosing the appropriate steroid preparation (ie, nonparticulate vs particulate), and using minimal or no sedation.
Image guidance is a must in the safe performance of these procedures. While planar fluoroscopy remains the imaging modality of choice at most institutions, CT guidance can be particularly helpful in cervical injections or in patients with challenging anatomy or prior surgery. Advantages of CT guidance include superior contrast resolution and primary axial plane guidance that allows direct visualization of target tissue and vulnerable structures along the proposed needle trajectory. Multisection CT fluoroscopy allows for target specificity with a similar dose to planar fluoroscopic guidance.7,8 While planar fluoroscopy provides the advantage of “live” intravascular injection detection using digital subtraction techniques, recent CT fluoroscopy studies have demonstrated vascular uptake detection rates similar to planar fluoroscopy.9,10 Although injection techniques using fluoroscopic or CT guidance are similar, there are few head-to-head studies assessing the effectiveness and safety of injections performed using CT versus fluoroscopy.
This edition of the AJNR News Digest highlights several recent spine pain management articles focusing on injection safety, technique, pitfalls, and novel procedures. In an elegant study using flat panel catheter angiotomography, Gregg et al11 document the intraforaminal location of thoracolumbar radicular arteries providing an anterior radiculomedullary artery. The location of these vessels within the neural foramen is relevant to needle placement during TFESI. Inadvertent injection or injury of branches contributing to the supply of the anterior spinal artery during TFESI can result in spinal cord damage and can lead to paralysis; therefore, targeting the “safe triangle” between the pedicle and nerve root, as has previously been advocated, to avoid nerve injury during needle placement may actually increase risk of intravascular penetration.12 Retroneural (commonly performed using CT) or infraneural techniques are increasingly used during the performance of lumbar TFESI to avoid the risk of vascular penetration. These techniques are likely safer without compromising efficacy.13
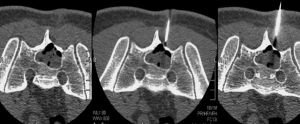
Posterior circulation stroke and cervical cord infarcts have been reported as extremely rare but potentially devastating complications of cervical TFESI, likely related to inadvertent intravascular injection of steroids or direct arterial injury.14 In a second study featured in this Digest, Lagemann et al15 report on the safest needle tip position during CT-guided cervical transforaminal epidural steroid injections, as determined by the incidence of intravascular injection. In their study, intravascular injections occurred in 24% of procedures and were lowest with an extraforaminal needle tip position. It is worth noting that in this study and other similar studies, negative aspiration did not exclude intravascular uptake.16 While Lagemann et al describe the safest position using an anterior approach, other authors have advocated using a dorsal approach for cervical TFESI.17 Future studies are needed to assess outcomes (ie, pain relief) in relation to needle tip position and intravascular injection incidence.
With the exception of 1 recent report,18 observations of catastrophic neurologic complications after the performance of TFESI reported so far have involved the injection of particulate steroids. The exact mechanism of injury is not well-understood but is thought to be due to the embolization and occlusion of end arterioles. Particulate corticosteroids, including methylprednisolone acetate, triamcinolone acetonide, and betamethasone acetate, contain corticosteroid esters that are insoluble in saline, local anesthetic, and iodinated contrast material.19 Methylprednisolone has the largest particle size, whereas betamethasone has the smallest.19 Animal studies have demonstrated ischemic lesions and hemorrhagic necrosis involving the spinal cord and posterior fossa in rats20 and pigs21 after direct injection of particulate steroids into the internal carotid or vertebral arteries, but no lesions or deleterious effects with intra-arterial injection of a nonparticulate steroid (eg, dexamethasone).
This discussion brings us to another article that helps us understand 1 potential mechanism of intra-arterial injury with particulate steroids. Laemmel et al22 used an in vivo mouse model and conducted in vitro experiments on human red blood cells (RBCs) to elucidate mechanisms of vascular compromise when steroids are intra-arterially injected. In their study, particulate steroids (eg, prednisolone, methylprednisolone, and triamcinolone) caused immediate and complete cessation of capillary blood flow due to the formation of RBC aggregates associated with the transformation of RBCs into spiculated RBCs. Dexamethasone did not alter microvascular blood flow or cause RBC agglutination or transformation. This elegant study provides us with valuable insights into particulate steroid-induced vascular compromise. However, more studies are needed to assess the safety of other therapeutic agents.
As part of an FDA safe use initiative, an expert multidisciplinary working group has outlined safety guidelines to prevent neurologic complications resulting from epidural steroid injections.23,24 The group recommends that particulate steroids should not be used in cervical TFESI and that a nonparticulate steroid should be the first-line agent for lumbar TFESI. Nonparticulate corticosteroids have rapid onset but short-lived anti-inflammatory effects, whereas particulate corticosteroids are thought to have delayed but sustained effects. This brings us to an important question: Are nonparticulate steroids less effective than particulate steroids in the performance of TFESI? While several published studies25-27 have shown that dexamethasone may not be less effective for improving pain and function than particulate steroids for TFESI, some of the prospective studies are underpowered. Additional studies with larger numbers of patients may provide more insight. However, owing to its better safety profile, many practices now only use dexamethasone for all TFESI.
Our discussion now moves from procedural safety during TFESI to recognizing unexpected contrast spread patterns during the performance of ILESI. Inadvertent intrafacet injection can occur during ILESIs via injection into the retrodural space of Okada, resulting in a false-positive loss of resistance and nontarget injection of medicine. The next article in this edition by Kranz et al28 provides insight into this fascinating potential space. A communication pathway between single-level bilateral cervical facet joints was first described by Dr. Kikuzo Okada in 1981.29 He found that 80% of the studied cervical facet joints could communicate with the interspinous soft tissues and contralateral facet joints via an extradural space that lies dorsal to the ligamentum flavum. This potential space, referred to as the retrodural or retroligamentous space of Okada, can act as a conduit for the spread of inflammatory or infectious processes along the posterior ligamentous complex.30 Kranz et al28 identified inadvertent intrafacet injection during CT fluoroscopic-guided ILESI at a rate that is 10-fold greater (7.5% of cases) than that observed for the same procedure performed under conventional fluoroscopy guidance. The low number of injections detected using planar fluoroscopy may be due to underrecognition of intrafacet injections during the performance of ILESI.31 Awareness of this potential space during spine injection procedures is therefore important to prevent the unintended spread of injectate into nontarget tissues.
This Digest also highlights novel treatments for challenging causes of spinal pain. In the penultimate paper of this edition, Murphy et al32 report on the effectiveness of their previously described CT-guided, 2-needle aspiration and fibrin injection technique to treat 213 consecutive patients with symptomatic Tarlov cysts.33 Tarlov cysts are common, incidental findings on lumbosacral MRI.34,35 While these are usually asymptomatic, depending on their size and location, they occasionally may cause symptoms including axial sacrococcygeal pain, perineal pain, sensory loss, radiculopathy, and bowel/bladder/sexual dysfunction.36 Surgical treatment can be effective but is not benign.37 Murphy et al32 show their percutaneous technique to be an effective and safe long-term treatment option.
The final paper in this Digest by Bellini et al38 describes an intradiscal treatment for symptomatic disc herniations. The authors performed chemonucleolysis in 80 patients with lumbar or cervical disc herniations that were refractory to conservative treatment using radiopaque gelified ethanol administered under fluoroscopic guidance. At 3-month follow-up, a substantial number of treated patients had symptomatic relief without clinical side effects. Discogenic pain can be difficult to diagnose and treat, and intradiscal therapies (including biacuplasty and regenerative therapy), despite limited evidence of efficacy, offer some early promise.39