Zhicai Chen |
 JianZhong Sun |
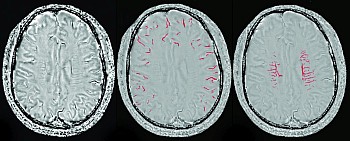
Intravenous thrombolysis with recombinant tissue plasminogen activator is a proved treatment for acute ischemic stroke within 4.5 hours of symptom onset. The foundation of intravenous thrombolysis for ischemic stroke involves reperfusion of the ischemic penumbra and salvage of threatened but potentially viable brain tissue. Work on imaging of the penumbra and clinical selection using penumbral evaluation have been one of the major focuses of our group. Despite the promise offered by PWI and DWI MR studies to advance patient selection for reperfusion therapies beyond noncontrast CT, sensitivity and specificity were still low. There is no consensus about the optimal perfusion parameter to accurately define ischemic tissue, and it is uncertain whether perfusion lesion volumes overestimate the penumbra tissue. So, there remains a thirst to develop new techniques that may potentially represent the penumbra based on the cerebral pathophysiology of an individual patient.
Recently, the use of blood oxygen level-dependent (BOLD) imaging as an alternative to DWI-PWI mismatch has stirred a lot of interest. It is sensitive to an increased concentration of deoxyhemoglobin (DHb) and may therefore be an indirect marker of oxygen metabolism. There are two approaches to